A team of researchers led by Professor Jonathan Rocheleau (BME), has introduced an innovative biosensor, Apollo-NADP+, in living zebrafish embryos to track molecular metabolism. This work opens new pathways to cancer and diabetes treatments.
The new study, published in Science Advances, explores the potential applications of this technology in understanding cellular processes, while addressing a critical question in diabetes research.
The Apollo-NADP+ sensor was engineered to detect changes in NADPH/NADP+ redox state (i.e., a chemical battery) as changes in fluorescence anisotropy. Unlike conventional methods of analyzing substances, which require cell fixation and destruction of biological samples, this sensor allows for real-time imaging of nutrient-stimulated metabolism, providing researchers with insight into the cellular dynamics of insulin secretion, which is directly relevant to the pathogenesis of diabetes.
“We’ve developed a tool that enables us to visualize cellular processes with high spatial-temporal resolution,” explains Cindy Bui (BME PhD candidate), a lead researcher on the study.
“This sensor has the potential to revolutionize our understanding of redox state in various cell types, with significant implications for fields such as cancer and diabetes research.” The study’s first phase focused on demonstrating the sensor’s functionality within living zebrafish embryos.
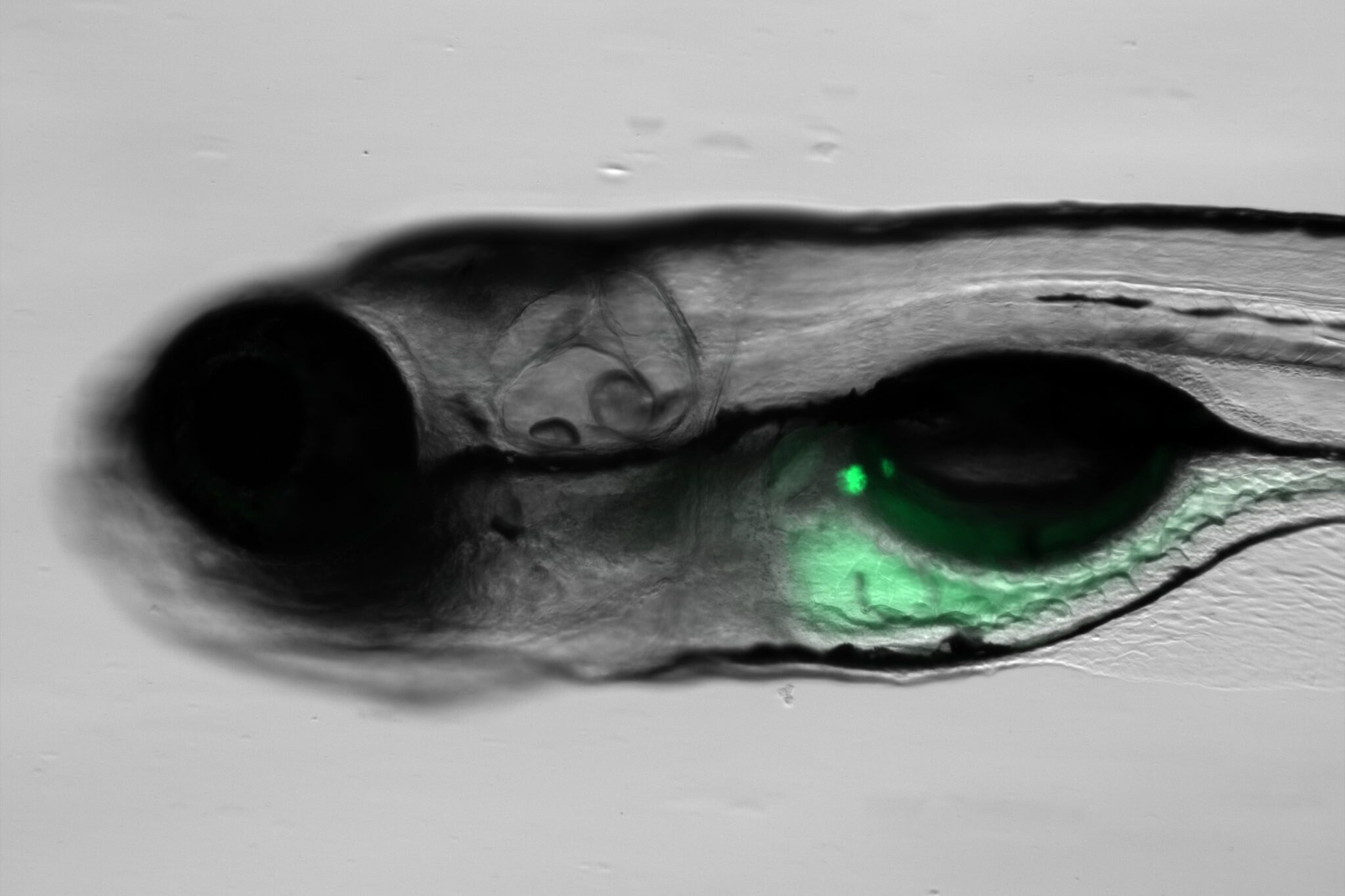
By inserting DNA encoding the sensor into the genome, researchers were able to establish a stable line of zebrafish that expresses the fluorescence protein-based sensor in the pancreatic islet. This breakthrough, achieved by working in collaboration with Dr. Brian Ciruna’s laboratory at The Hospital for Sick Children (SickKids), enables possibilities for studying cellular processes in a dynamic, live environment.
Further investigations delved into the redox state of pancreatic beta cells, a critical area of interest in diabetes research. The team found evidence suggesting that stressed beta cells utilize alternate pathways for NADP+ reduction, challenging previous assumptions about their metabolic processes.
“This research not only reveals the versatility of the family of Apollo sensors to work in vivo but also uncovers potential implications for diabetes treatment strategies,” says Rocheleau, the principal investigator of this research.
“Understanding how beta cells modify their metabolism in response to oxidative stress may uncover new avenues for therapeutic intervention.”
The study’s findings offer a versatile tool for researchers across various disciplines, adds Rocheleau, including the ability to monitor cellular processes in real-time, without the need for cell fixation. This can lead to a wide range of potential applications in both basic science and clinical research.


