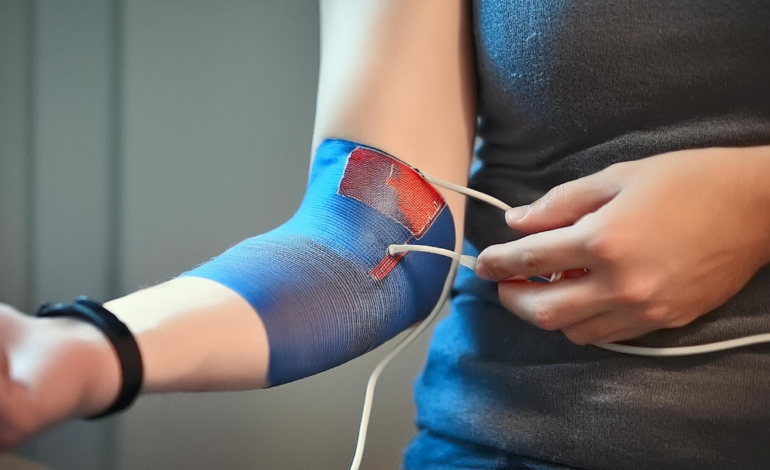
A recent study published in BioMedical Engineering OnLine introduces a novel smart sleeve designed for Functional Electrical Stimulation (FES) applications. This new technology integrates advanced carbon-based dry electrodes within a textile structure, offering significant improvements in comfort and usability for patients with motor control impairments.
This study was led by Baptiste Garnier, with contributions from Melissa Marquez-Chin, Stephanie DiNunzio, Stephanie Iwasa, Zia Saadatnia, Dr. Hani E Naguib, and Dr. Milos Popovic. This study was a collaboration between KITE-Toronto Rehabilitation Institute, the Institute of Biomedical Engineering at the University of Toronto, the Department of Mechanical & Industrial Engineering at the University of Toronto, and the Department of Mechanical and Manufacturing Engineering at Ontario Tech University.
Functional Electrical Stimulation is a rehabilitation technique that sends low-energy electric pulses through peripheral nerves to activate muscles in paralyzed individuals. Despite its effectiveness, traditional FES systems using hydrogel electrodes and long cables are cumbersome and limit practical applications. To address these challenges, the research team developed a wearable smart sleeve that simplifies electrode application and enhances patient comfort.
The smart sleeve, equipped with dry electrodes, offers several advantages over traditional hydrogel electrodes. First, it provides improved comfort, as participants reported lower discomfort ratings across different intensity levels. Second, the integration of electrodes within a textile substrate simplifies wearing and removal, which is especially beneficial for individuals with impaired hand function. Third, the dry electrodes effectively stimulate muscles, comparable to hydrogel electrodes, without significant differences in muscle torque. Finally, the materials and processes used for the smart sleeve are commercially available, making it feasible for scalable production and widespread clinical adoption.
This innovative approach offers a practical solution for delivering FES therapy outside of clinical settings, making it more accessible and user-friendly for patients and healthcare providers. The wearable nature of the device makes it suitable for long-term applications, potentially revolutionizing rehabilitation for stroke and spinal cord injury patients.
Further studies are planned to evaluate the long-term durability and mechanical robustness of the smart sleeve. Additionally, clinical trials with a larger participant pool are underway to gain regulatory approval and facilitate broader dissemination of this technology.