Researchers from IBBME and Faculty of Dentistry have recently uncovered an alternate way in which the cells communicate with one another during wound repair. The findings have important implications on the development of wound healing therapies.
“What we found is that wound healing cells respond to mechanical signals,” says Pardis Pakshir, the leading author on the Nature Communications paper published in April this year. “We are establishing a new form of communication between these cells that has not been shown before.”
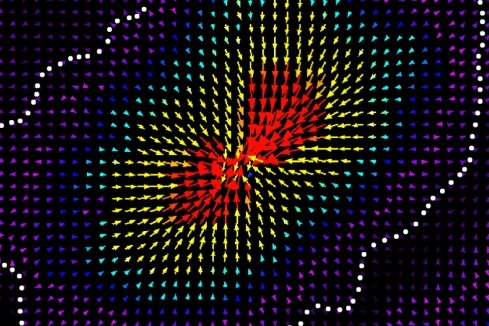
During wound repair, tissue cells produce a network known as the extracellular matrix, which acts as a scaffold platform for cells to come together. Two important players during wound healing are fibroblasts and macrophages. The fibroblasts are responsible for generating the extracellular matrix, while macrophages participate in the wound healing process.
“One way to think about these extracellular matrices is to imagine them like a series of interconnected ropes.” says Pardis, “Fibroblasts are known to hang on to these ‘ropes’, and literally pull on them. What we found is that their pulling attracts macrophages that are sitting a few millimeters away on the same ‘ropes’ to come closer.”
Normally, macrophages migrate in response to a chemical signal sent out by other cells. What’s different about the findings here is that wound healing macrophages respond to mechanical forces – in this case, the ‘tugging’ signal from fibroblasts.
By growing both the macrophages and fibroblast cells together in 2D and 3D cell tissue models, Pardis and her colleagues were able to manipulate the individual cells to figure out the mechanism of action. Their collaborators in the international team then used computational simulations to provide additional sources of evidence to validate their hypothesis.
These findings have significant implications on how other researchers design wound healing materials that responds to the mechanical signals in the body.
“If you are designing a bio-material that is going onto the valve of a heart as a sealant or to cover the wounds of a burn patient, you’d have to consider the mechanical properties of the material. For example, what kind of tension does it create? By taking these parameters into consideration, you can design better bio-materials that respond to the mechanical properties of the body.” says Pardis.
“This study is a great example how the IBBME brings together researchers from different disciplines and faculties to create synergies and innovations that would otherwise be difficult to achieve. Like the cells in our study, no researcher is an island.”, says Dr. Boris Hinz, the corresponding author of this publication.